pH

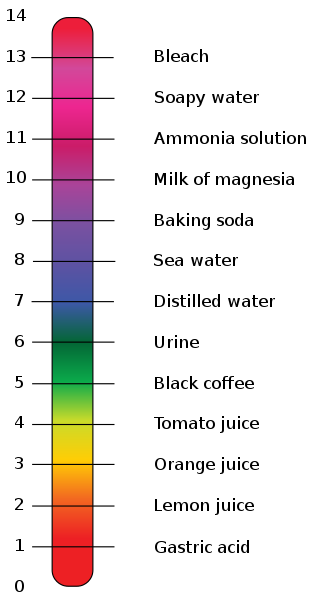
In chemistry, pH is a measure of the activity of the (solvated) hydrogen ion.
Pure water has a pH very close to 7 at 25°C. Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline.
The pH scale is traceable to a set of standard solutions whose pH is established by international agreement. Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode. Measurement of pH for aqueous solutions can be done with a glass electrode and a pH meter, or using indicators.
pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, and many other applications.